 *Image taken from the public domain. A new type of bacteria that can only survive with caffeineIf you think you have seen it all, this blog entry will blow your mind. Researchers from the University of Austin, Texas, engineered a strain of Escherichia coli that can only survive with caffeine. Yes, caffeine, the holy grail of graduate students. But, why in the world would someone want to engineer a microorganism that would be capable of metabolizing caffeine? It turns out that, with the continuous growth of pharmaceuticals and other industries all around the world, there is an increase of caffeine as a pollutant in the water and land around cities. So, it was necessary to develop a strategy that would help diminish the quantity of caffeine from the environment.
As weird as it seems, nature had a way of solving this pollution crisis. The recently discovered bacteria, Pseudomonas putida CBB5, was found to be able to metabolize caffeine in the environment. By analyzing the metabolic network of P. putida CBB5, researchers discovered which enzymes were necessary for digesting caffeine. So, they used this information to engineered E. coli in a way that it can produce the enzymes that are necessary for metabolizing caffeine.
There you have it. In the future, we could be able to use these bacteria to produce decaffeinated beverages, in addition of cleaning the environment. Could these new discoveries benefit the Caffeine Beverage Industry? Who knows...*References:(1) http://www.popsci.com/science/article/2013-03/bacteria-caffeine-addict-its-possible
 *Image from the public domain | The secret of bacterial alchemistsAs taken from a fantasy novel, nature surprises us one more time with a marvelous story. This is the case for two species of bacteria, the extremo-philes Cupriavidus metallidurans and Delftia acidovorans. Using a metagenomics approach, researchers from the University of Adelaide, discovered these two species of bacteria forming biofilms on the surface of gold grains from a mine in Queensland, Australia. What is interesting about these two species is that they are capable of transforming liquid gold, in the form of the toxic gold chloride, into solid 24-karat gold. Yes, in pure solid gold.
One of the most precious and expensive materials in our planet can be transformed from a liquid form to a purer solid state by bacteria. | And not just that, these bacterial species were also found to dissolve gold grains into nanoparticles that move through rocks and crevices, thus contributing to the movement of gold around the environment. In addition, this natural phenomenon contradicts the belief that gold can only be formed through physical geological processes.
This unprecedented alchemical process had fascinated scientists all around the world due to its unique nature. Who would have thought that microorganisms could be capable of transforming gold to a pure solid state? Once again, nature has shown us how surprising is our planet. I am certain that, in a near future, microbes will surprise us once again with an incredible discovery. That is why I am counting on metagenomics as a tool to expand our knowledge in this microbial planet that we live in.*References:(1) Bacteria Make Gold Nuggets, Discovery Magazine(2) Gold-Loving Bacteria Show Superman Strength, Michigan State University News  *Gold granules produced by the bacterium Cupriavidus metallidurans. Photo by G.L. Kohuth. Taken from the work of Adam W. Brown and Dr. Kazem Kashefi, Michigan State University.
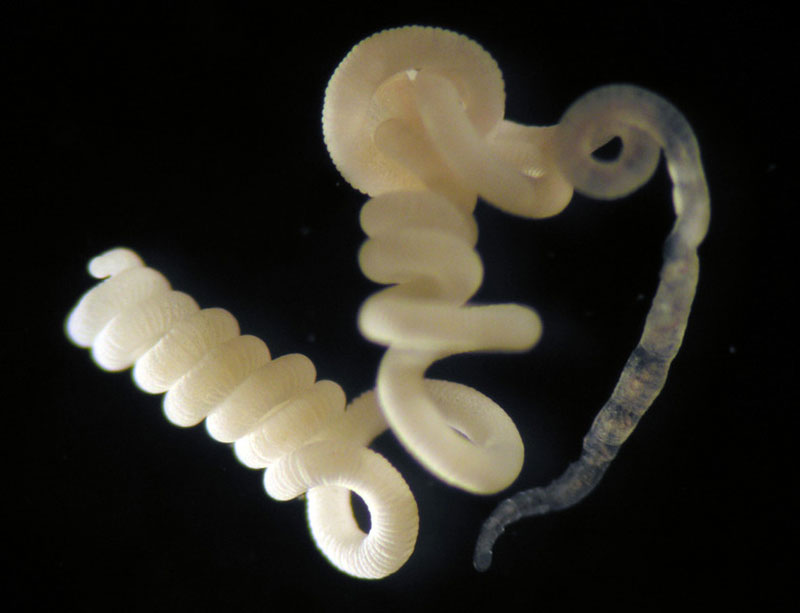 *Olavius algarvensis | Image from C. Lott/ HYDRA/ Max Planck Institute for Marine Microbiology, Bremen. A poisonous food for a gutless animal
In the sandy sediments off to the coast of the Mediterranean island of Elba in Tuscany, Italy, resides an amazing oligochaete worm, Olavius algarvensis. This marine worm is very peculiar because of its anatomy and physiology. What is interesting of this animal is that it completely lacks a digestive tract; no mouth, no stomach, no intestines. However, it remains as a very functional animal within its ecosystem. But what is weird of this marine worm is that its diet is composed of very toxic compounds like carbon monoxide and hydrogen sulphide. So, how in the world, an animal with no digestive tract can survive by consuming solely toxic compounds? It turns out that a group of bacteria is responsible for their nourishment, their waste management, and their survival.Knowing how the endosymbiotic bacteria help nourish and maintain the nutrition demands of this marine worm was a mystery for scientists around the world. However, on 2006, a group of scientists from the U.S. Department of Energy Joint Genome Institute (JGI), in collaboration with other institutions, published a paper in which they described, using a metagenomics approach, how outsourcing energy and waste management was maintained in the marine worm by their endosymbiotic bacteria. Their goal was to obtain all the genomic content of the endosymbiotic bacteria that resided in all the body parts of the marine worm Olavius algarvensis. After shotgun sequencing of the genomic data of all the metagenomes of the bacteria that resided within O. algarvensis, they were able to identify genes that had a functional role in metabolic pathways that were involved in management of waste products and energy outsourcing. Their analysis revealed how dependent marine worms were from their endosymbionts. Nevertheless, they found evidence suggesting that their symbiotic bacteria are not obligate host-dependent and could survive outside the host in a free-living stage.This study was of important value to science, specially to symbiosis research because it was the first instance in which a metagenomic shotgun sequencing approach was used to study mutualistic interactions between bacteria and their host. With studies like this, we can learn more about successful strategies of adaptation that many animals have in our planet. Don't forget to watch the video at the end of this blog entry! 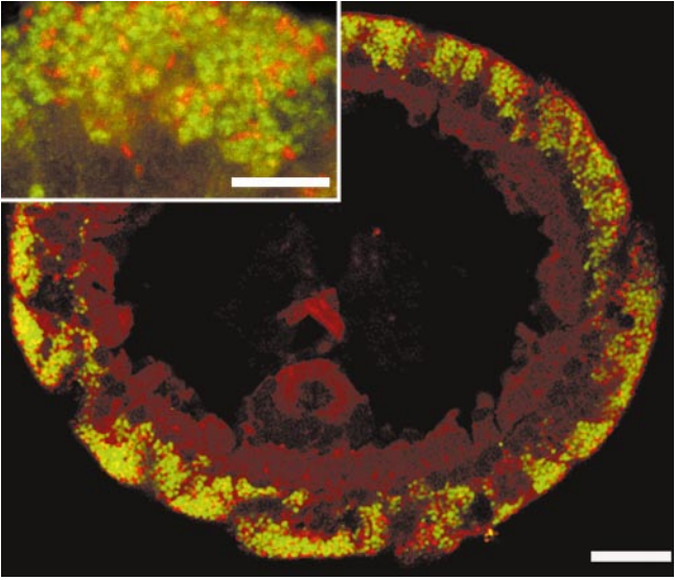 *Fluorescence in situ hybridization of endosymbionts in O. algarvensis. Obtained from Dubilier et al. (2001) Nature
|





 RSS Feed
RSS Feed